Elevate Your Expectations.
Identify Accurate Tests.
Avoid Over & Under Treatment.
Your Indispensable Partner in Evaluating Laboratory Test Quality

Elevate Your Expectations.
Identify Accurate Tests.
Avoid Over & Under Treatment.
Your Indispensable Partner in
Evaluating Laboratory Test Quality
ELEVATEGENETICS® is Maximizing Value in Precision Medicine By Promoting More Accurate Genetic & Genomic Testing, Analysis, & Reporting
- Hereditary Cancer Panels
- Comprehensive Genomic Profiling for Oncology (CGP)
- Carrier Screening Panels
- Rare Disease Panels
- Cardiac Disease Panels
- Whole Exome Sequencing (WES)
- Pharmacogenomic Testing (PGx)
- Non-invasive Prenatal -Testing/Screening (NIPT/NIPS)
- Polygenic Risk Scores (PRS)
- Gene Signature Tests
- Prognostic Genetic/Genomic Tests -Artificial Intelligence (AI) Tests
- Pharmacogenomic Testing (PGx)
- Hereditary Cancer Panels
- Comprehensive Genomic Profiling for Oncology (CGP)
- Carrier Screening Panels
- Rare Disease Panels
- Cardiac Disease Panels
- Whole Exome Sequencing (WES)
- Pharmacogenomic Testing (PGx)
- Everything in clinical genetics and genomics
The Problem
Not all clinical laboratory tests are sufficiently accurate.
Genetic and genomic tests with seemingly impressive technical specifications and laboratory accreditations may not be as accurate as they appear. This is because regulation, standardization and transparency expectations for laboratory developed tests lags far behind what is needed for stakeholders to distinguish between clinically valid and invalid tests.
Inaccurate genetic and genomic test results can oftentimes lead to inappropriate downstream medical care that can:
- Harm patients
- Increase wasteful spending in the healthcare system
While no diagnostic test is perfect, efforts must be made to protect patients from diagnostic tests with avoidable and harmful flaws that can be discovered through leading edge independent evaluation.
The Solution
Use ELEVATEGENETICS® services to find the most accurate genetic and genomic tests.
The independent and patient-focused nonprofit organization, Center for Genomic Interpretation (CGI) provides unique and cutting edge accuracy and quality assessment services to help stakeholders discover the most accurate and clinically valid genetic and genomic tests. No other organization provides such powerful services. By doing so, CGI’s ELEVATEGENETICS services assist in:
- The promotion of better clinical outcomes for patients
- Improvement of financial outcomes for an already overburdened healthcare system
ELEVATEGENETICS picks up where CLIA/CAP accreditation leaves off.
ELEVATEGENETICS helps you identify the most accurate tests.
“We believe this is a viable and credible way to ensure the labs’ quality and accuracy of testing.”
– Blue Shield of California, a CGI customer

BRILLIANT
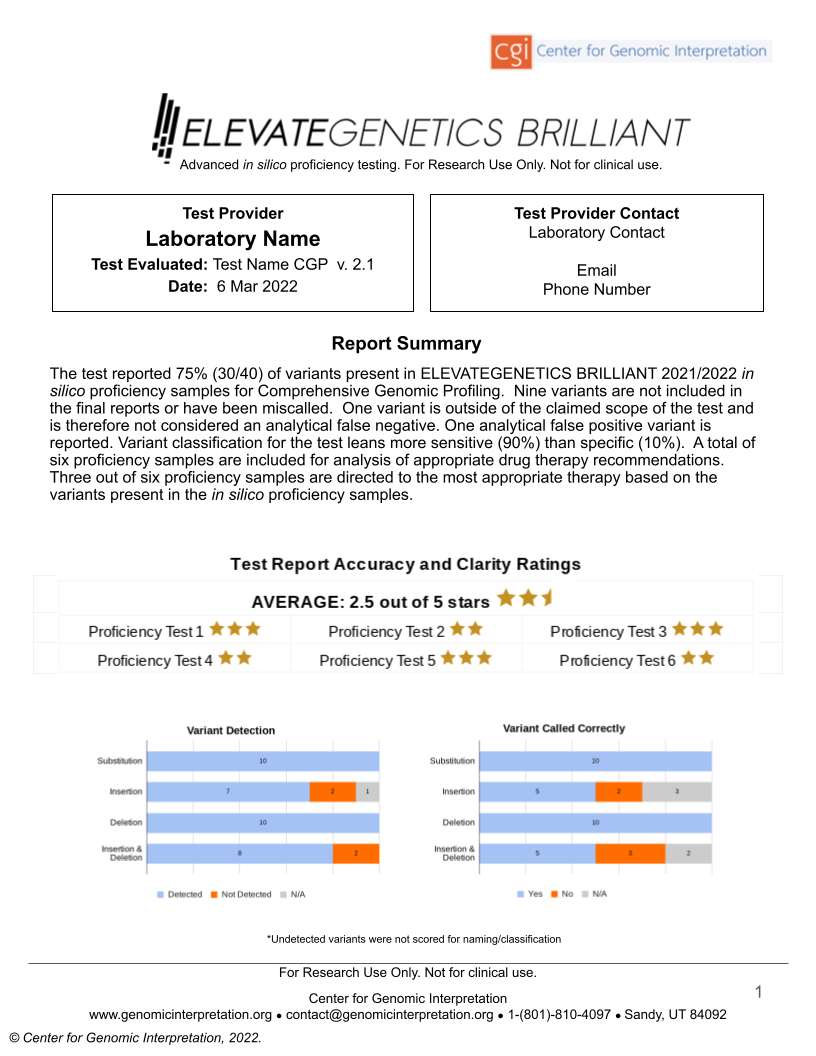
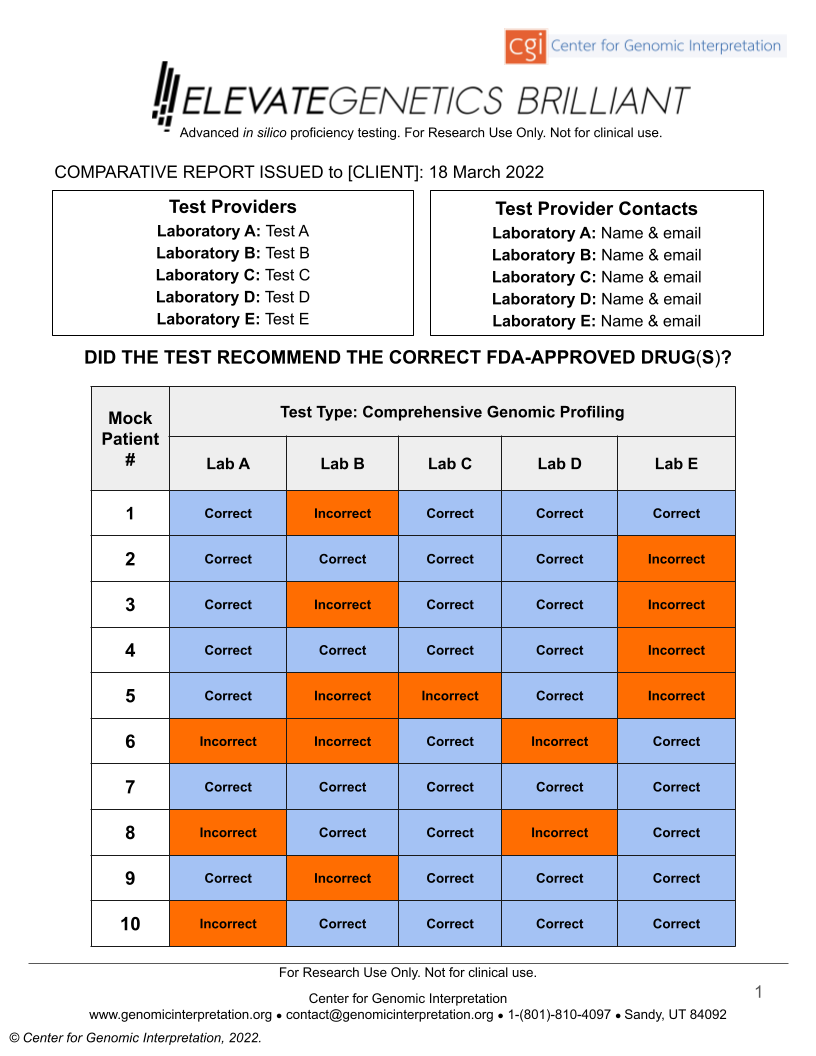
ELEVATEGENETICS BRILLIANT™ is a performance evaluation on mock patients which scores Next Generation Sequencing (NGS) tests for their propensity to correctly identify and report actionable mutations and biomarkers. This will minimize false negative and false positive test results that may lead to inaccurate care decisions.
- Variant detection
- Variant naming
- Variant classification
- Lab-issued report clarity
For the first time since the advent of NGS, stakeholders can now compare* standardized performance metrics and can clearly and confidently identify strengths and weaknesses of different genetic and genomic tests through the use of ELEVATEGENETICS BRILLIANT.
*With permission from participating laboratories.
“Most genetic tests today are not regulated, meaning that they go to market without any independent analysis to verify the claims of the seller.”
“Regulation of Genetics Tests”. National Institutes of Health – National Human Genome Research Institute.
https://www.genome.gov/about-genomics/policy-issues/Regulation-of-Genetic-Tests


VERIFY
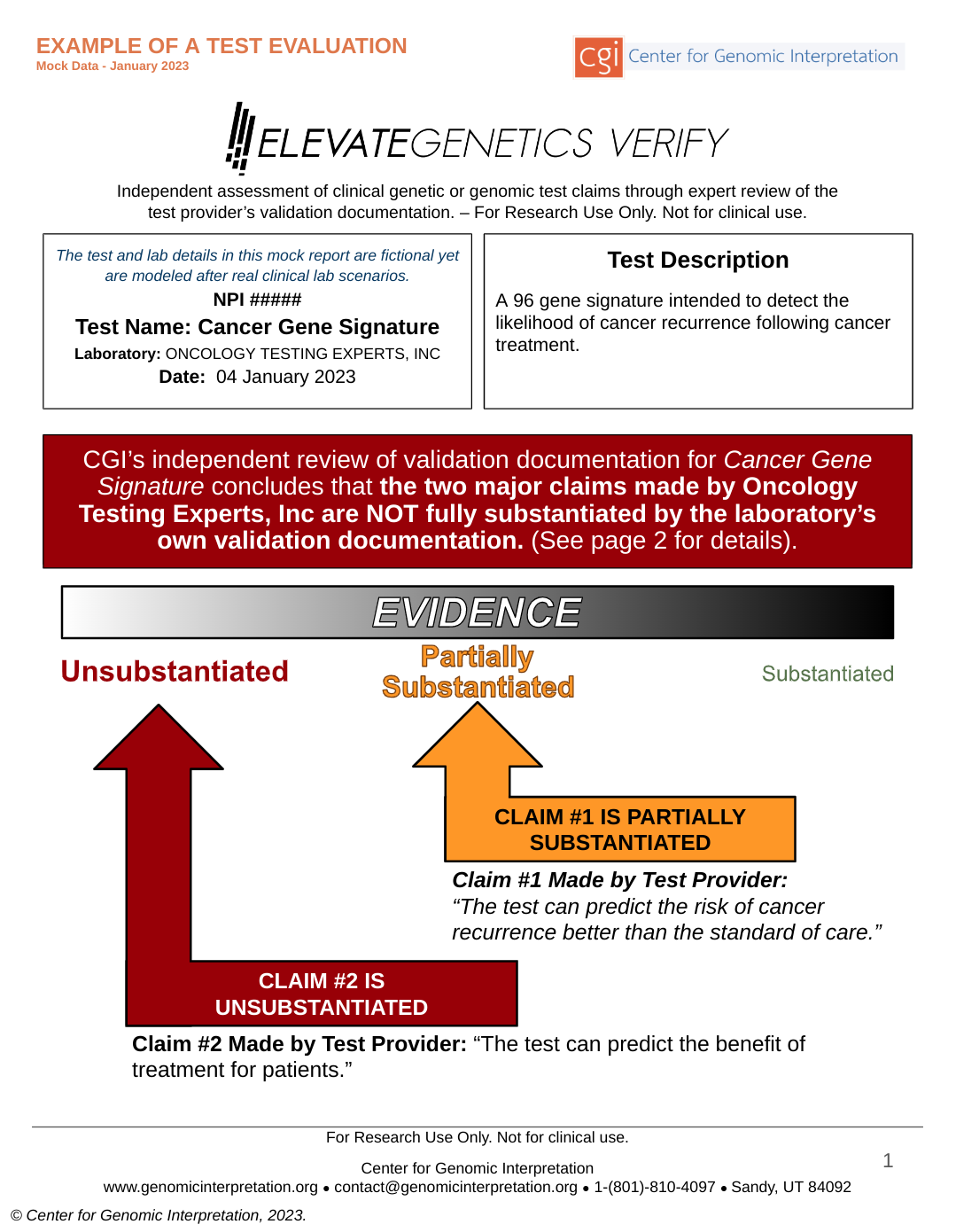
ELEVATEGENETICS VERIFY™ is an independent and expert assessment of clinical genetic/genomic test claims through a detailed review of the test provider’s validation documents. This will assist stakeholders in assessing if the test is able to perform as claimed.
ELEVATEGENETICS VERIFY™ can be requested by payers for novel or complex genetic and genomic tests, particularly those incorporating polygenic risk scores, complex algorithms, or artificial intelligence. Clinical laboratories can also complete this review at their own expense and provide the results to payers.
ELEVATEGENETICS VERIFY can find the inappropriate shortcuts, as well as mistakes that laboratories sometimes make when validating their own tests. These errors can result in tests that give frequent, inaccurate results to patients. Some of the most common errors laboratories make when validating their tests are:
- Underpowered studies
- Insufficiently matched cases and controls
- Overfitting by using samples run in development as validation samples
- Over-reliance on non-realistic validation samples
ELEVATEGENETICS VERIFY reports are simple and easy to understand. They succinctly describe which of the test seller’s claims have been substantiated by validation studies, and which have not.
“Upon reviewing validations, we’re refusing to reimburse 50% of these tests because we don’t think that the lab performance characteristics are good enough.”
–Payer from Tapestry Networks Diagnostic Quality Assurance Pilot, Summary of Themes, March 22, 2021.

CLARITY
ELEVATEGENETICS CLARITY™ supports prior authorization and care coordination when certain precision therapies are recommended based on a positive genetic or genomic test result.
ELEVATEGENETICS CLARITY can be used by payers or physicians to help determine whether a downstream medical intervention is likely to improve outcomes for a patient based on evidence supporting the variant classification.
The classification of variants identified on the patient’s genetic or genomic test report is fact checked by experts through a careful assessment of existing scientific findings, which helps provide a clearer picture of appropriate next steps.
Applications for ELEVATEGENETICS CLARITY can include assisting in prior authorizations for:
- Gene therapies
- Approval of oncology precision therapeutics
- Risk-reducing surgical interventions associated with inherited disorders
“The most significant contributor to false positive test results is imperfect variant classification…”
“A problem for the industry is that…[the same] variant is classified as a ‘variant of unknown significance’, ‘likely benign’, and ‘pathogenic’ by different laboratories due to variations in interpretation.”

LANDSCAPE
ELEVATEGENETICS LANDSCAPE™ evaluates the billing accuracy of a genetic or genomic test administered by a laboratory based on the technical specifications of that specific test and/or laboratory.
From a quality perspective, this represents a potential source of false negatives.
From a billing perspective, the laboratory is billing for tests it has failed to deliver in full.
ELEVATEGENETICS LANDSCAPE is more foundational than a billing code specificity analysis. In a specificity analysis, the payer will determine if the most specific billing code(s) were used by the laboratory for the test they describe.
In contrast, LANDSCAPE checks to make sure that the test the laboratory describes to the payer is even possible based upon the application of the laboratory’s technologies.
ELEVATEGENETICS LANDSCAPE can be used as a standalone quality check or as a precursor to a billing code specificity check. In addition, LANDSCAPE also pairs well with ELEVATEGENETICS BRILLIANT to evaluate sequencing tests’ validity and usefulness.