Why CGI?
To better answer this question, let’s first look at a timeline of Laboratory Developed Tests (LDTs) & regulation (or lack thereof) within the genetic and genomic testing industry
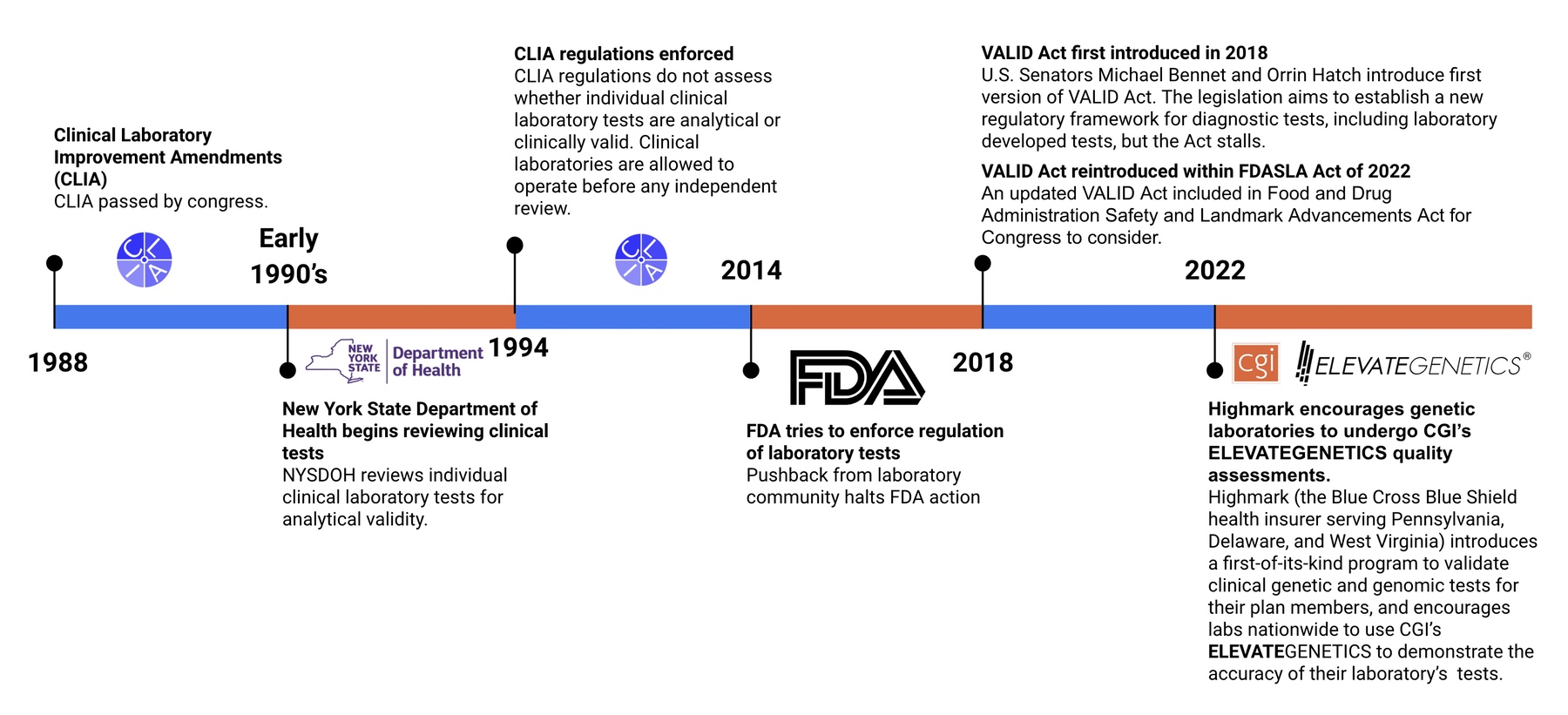
You may be wondering…
What are the differences between each of these organizations?
Why do we need multiple organizations that seemingly assess the same thing?
The best way to understand what each accreditation or regulation means when evaluating accuracy and improving patient care is to explain what is assessed by each accrediting organization.


CLIA accreditation and CAP (and other institutions, listed here) accreditation are connected. That is, CAP inspections are solely sufficient to meet both CLIA and CAP accreditation requirements.
Due to this relationship, the limitations of CLIA and CAP inspections are often related. However, laboratories are allowed to test patients without CAP or other accreditations and potentially receive insurance reimbursements. This could be the case when an independent CLIA survey is performed.
Limitations
CAP accreditation is focused on laboratories and departments as a whole rather than scrutinizing individual test offerings.
This means that a laboratory can choose to only show documentation to an inspector for their highest performing and best documented genetic or genomic test(s) to meet CAP checklist requirements, which is NOT a guarantee that every test offered by a laboratory meets the same standards.
CAP accreditation is largely based on checklist requirements. Checklist requirements primarily focus on the presence and absence of documentation and less on the exact details of the documentation and the contained information.
Real world example:
A checklist requirement states that a validation document should exist and that this document has information about the sensitivity of a test, but the details of what the sensitivity ultimately is and how it compares to existing tests from other laboratories is NOT assessed.

It is possible for several tests intended to diagnose the same condition to have markedly variable performances and standards of evidence and still be marketed with the same CAP accreditation.
CAP accreditation reviews are not always independent. CAP inspections from third-party reviewers only happen once every two years. On the “off” years, CAP inspections are performed internally by the laboratory.
It is easy to imagine how bad actors in the genetic and genomic testing industry could utilize these self-assessments to continue offering poor quality tests for two years without any third-party having any opportunity to assess the analytical or clinical validity of the test.
Although CAP does dedicate some checklist items to recording the analytical performance characteristics of the test, clinical validity is not thoroughly assessed by CAP checklist requirements.
There is limited evidence required to justify the inclusion or exclusion of certain genes and gene regions on genetic panels, resulting in tests that have limited diagnostic efficacy and produce clinically significant false positive results that impact patients.
The chart below outlines CLIA and CAP limitations before we move into other governing bodies scope of abilities and limitations, pertaining to genetic and genomic testing.

The New York State Department of Health (NYSDOH) issues permits by evaluating laboratories through the Clinical Laboratory Evaluation Program (CLEP). In addition to evaluating laboratories, laboratory developed tests (LDTs) are evaluated individually before patient samples from New York can be tested.
Although there are exceptions to this rule (example – NYSDOH-approved version of the requested test is not yet available), this policy has resulted in genetic and genomic tests receiving much greater scrutiny than they otherwise would through the CLIA/CAP accreditation process.
There are labs that are able to offer certain parts of testing for some patients and not others, due to lack of NYSDOH approval for the entire test offering.
Guidelines and checklists for test approval are publicly available at the NYSDOH/CLEP website.
Limitations
NYSDOH grants conditional approvals, which can mean the test has not been reviewed.
Although analytical characteristics are generally reviewed, clinical validity analyses are still limited compared to FDA review.

Instead of providing a list of checklist items that need to be included in a submission, the FDA publishes non-binding guidance documents that represent the agency’s current thinking on what should be assessed in a test validation (e.g. NGS guidance for germline disease).
The FDA submission and approval/clearance process is a more nuanced process than a strict set of rules that every genetic and genomic test must meet. Required testing and evidence can vary depending on the clinical context of the test and other factors that allow for flexibility in the process of assessing whether a genetic or genomic test is safe and effective.
Although the FDA does a thorough review of evidence for safety and effectiveness of the tests that they have approved or cleared, there are still potential limitations.
Limitations
FDA process for assessing that ALL genes on FDA-approved panels have demonstrated clinical utility (gene panel validity).
FDA confirming that variant classification in clinical practice does not lead to false positive results in de novo variant classification.
The Solution
Use ELEVATEGENETICSⓇ services to find the most accurate genetic and genomic tests.
The independent and patient-focused nonprofit organization, Center for Genomic Interpretation (CGI) provides unique and cutting edge accuracy and quality assessment services to help stakeholders discover the most accurate and clinically valid genetic and genomic tests. No other organization provides such powerful services. By so doing, CGI’s ELEVATEGENETICS services assist in:
- The promotion of better clinical outcomes for patients
- Improvement of financial outcomes for an already overburdened healthcare system
What is a laboratory-developed test (LDT)?
LDTs are non-FDA-approved or cleared assays that are developed and used within a single laboratory. FDA-approved or cleared kits that have been modified by a laboratory are also considered LDTs. Oftentimes, laboratories offer LDTs with the same intended use as available FDA-cleared or approved tests.
Although LDTs are often used in the same way as FDA-approved or -cleared tests, they are not regulated by the FDA simply because of where the tests are performed. This may seem an odd distinction, but historically, LDTs generally came from smaller, local laboratories and used to diagnose rare disease. Presently, the LDT landscape has changed completely with LDTs from a single location often marketed on a national scale. LDT designation is not related to anything besides the geography of where the test is performed, resulting in high-complexity and high-risk tests with:
- Claims that are not adequately supported by evidence
- Lack of appropriate controls
- Falsified data
Source: https://www.fda.gov/medical-devices/in-vitro-diagnostics/laboratory-developed-tests